 by Guneet Awal, MD, MBBS, and Simplepreet Kaur, MD, MBBS
by Guneet Awal, MD, MBBS, and Simplepreet Kaur, MD, MBBS
Drs. Awal and Kaur are with the Department of Dermatology, Venereology & Leprosy, Sri Guru Ram Das Institute of Medical Sciences and Research in Amritsar, India.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Neurodegenerative disorders such as Alzheimer’s disease and localized cutaneous macular amyloidosis are conditions that result from protein misfolding. These disorders share common pathogenic mechanisms that lead to the deposition of amyloid protein. Currently, there is a paucity of data on the connection between the brain and skin amyloidosis. Few recent studies have demonstrated a strong connection between the brain and skin in different amyloid diseases. Here, we report a case of concurrent occurrence of skin and brain amyloidoses and explore the brain-skin axis connection.
Keywords: Macular amyloidosis, Alzheimer’s disease, brain-skin axis
J Clin Aesthet Dermatol. 2018;11(4):25–27
Amyloidoses are a group of protein misfolding diseases in which the accumulation of protein aggregates occur either systemically or locally. In local amyloidosis, beta-pleated amyloid deposits are restricted to a particular organ.1 It is grouped into 1) neurodegenerative conditions where aggregation is seen in the brain and 2) non-neuropathic conditions in which other tissues are affected—for example, the skin.2 Alzheimer’s disease and cutaneous amyloidosis are the two conformational disorders in which a skin-brain connection has been established. Genes such as presenilin-1 and apolipoprotein E (ApoE4) play roles in amyloidogenesis in the skin and brain.3 Amyloidogenic material presenting outside of the central nervous system can have a direct impact in neurodegenerative amyloidosis. In this case report, we attempt to explain the correlation between brain and skin amyloidopathy.
Case Report
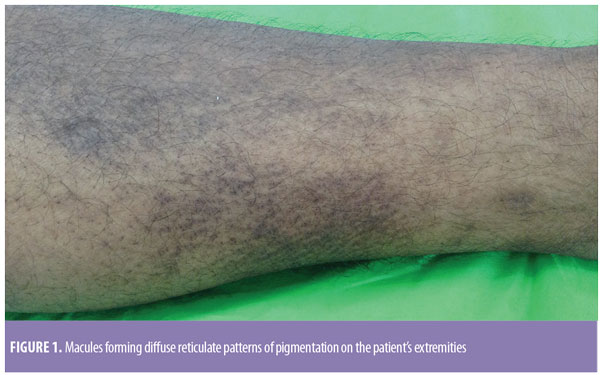
A 57-year-old male patient presented to the skin outpatient department with a seven-year history of hyperpigmentation and severe itching on the face, upper back, abdomen, and bilateral extensor aspects of the arms and legs. The feeling was associated with a thickening of the skin over the affected areas. On examination, dark brown-black irregular macules were present on the face, upper back, and abdomen. On the extremities, the macules had coalesced to form diffuse reticulate patterns of pigmentation (Figure 1). Concurrently, the patient had developed dementia, confusion, and aphasia. His Mini-Mental State Examination (MMSE) score was 11. On the basis of his MMSE score, the patient was labeled as having severe dementia with a probable diagnosis of Alzheimer’s disease. Consequently, a magnetic resonance imaging brain scan was advised, which showed prominence of the corticosulcal paces involving the bilateral temporoparietal lobes and prominence of both lateral ventricles suggestive of cortical atrophy, thus confirming the diagnosis of Alzheimer’s disease.
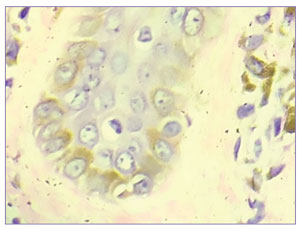
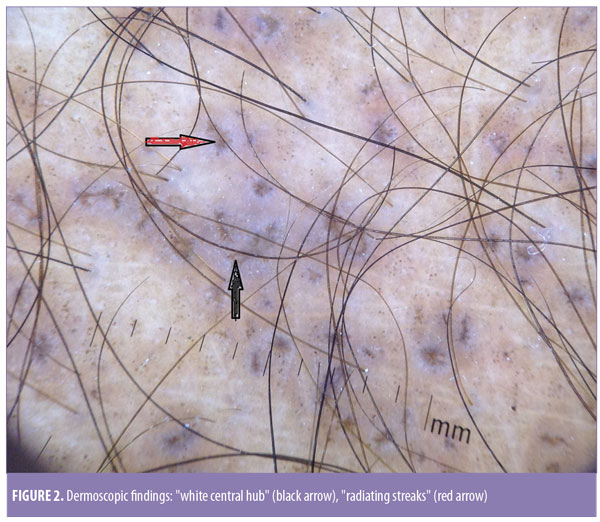
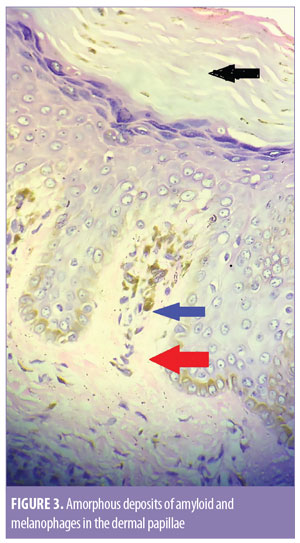
Dermoscopy was done using a DermLite® DL3N dermatoscope (3Gen Inc., San Juan Capistrano, California). A central white hub surrounded by radiating brown streaks was observed (Figure 2). On skin biopsy, the epidermis showed moderate compact and lamellated orthohyperkeratosis. Amorphous deposits of amyloid were observed in the dermal papillae, along with melanophages (Figure 3). Perivascular superficial sparse infiltration of melanophages was observed. This confirmed the diagnosis of macular amyloidosis.
The patient was also incidentally diagnosed with diabetes mellitus Type 2 and hypertension during his workup. His fasting blood sugar was 199mg/dL and hemoglobin A1c was 8.2. The results of other investigations, including complete blood count, thyroid profile, liver and renal function tests, urinalysis, and chest x-ray, were within the normal limits. Human immunodeficiency virus, hepatitis B, and hepatitis C were non-reactive.
Discussion
Amyloidosis is an extracellular deposition of fibrous protein either involving multiple organs or single areas of tissue.4 Amyloid, a cross-beta supersecondary structure that is deposited in tissues, results from the conversion of peptides or proteins from their soluble functional states into highly organized fibrillar aggregates.5 This is a complex process involving key players from the intracellular protein quality control system, extracellular chaperones and matrix components, proteases, and other cofactors.6 In localized amyloidosis, the deposition of amyloid can be seen in the brain in the form of neurodegenerative disorders and in the skin as primary cutaneous amyloidosis.2 Various neurodegenerative disorders that have been documented include Alzheimer’s disease, Parkinson’s disease, and amyotropic lateral sclerosis. Primary localized cutaneous amyloidoses can present as lichen, macular, or nodular amyloidosis.
In our patient, Alzheimer’s disease was concurrently present with macular amyloidosis. In this case report, we have tried to summarize the pieces of evidence that can be helpful in attempting to determine whether there is any link between skin and brain amyloidosis. Due to the paucity of data regarding this correlation, there is a need for further exploration. Alzheimer’s disease is clinically characterized by a progressive and gradual decline in cognitive function and neuropathologically characterized by the presence of neurofibrillary tangles and senile plaques.7 Diabetes is a risk factor for Alzheimer’s disease. Macular amyloidosis is clinically characterized by small, brownish macules with a characteristic reticulated or rippled pattern.4 In a study performed by Krishna et al,8 the interscapular area, one of the areas affected in our patient, was identified as the most common site of involvement. Additionally, Bandhlish et al4 concluded in their study that sun-exposed sites, such as the face and extremities affected in our case, are more commonly involved in macular amyloidosis.
Histopathologically, aggregates of homogeneous and eosinophilic dermal deposits are seen. On staining with Congo red, under polarized light, apple green birefringence is observed.9 In the brain, beta amyloid deposits are seen initially in the parahippocampal region and later in the limbic system.3
A study done by Clos et al2 revealed that there might be a connection between the cutaneous form of amyloidosis and neurodegenerative amyloidosis. Amyloid deposits in the brain and skin share a common ultrastructure and pathogenic mechanism. The cross-seeding mechanism in the skin that leads to amyloid deposits in the brain through a misfolded form of ?-synuclein indicates that the presence of amyloidogenic material outside the brain can have a direct impact on neurodegenerative amyloidosis.2 A specific amyloidogenic peptide sequence has been identified in the skin and brain. Furthermore, the basic pathogenic mechanisms for skin and brain amyloidopathy seem to be similar.3
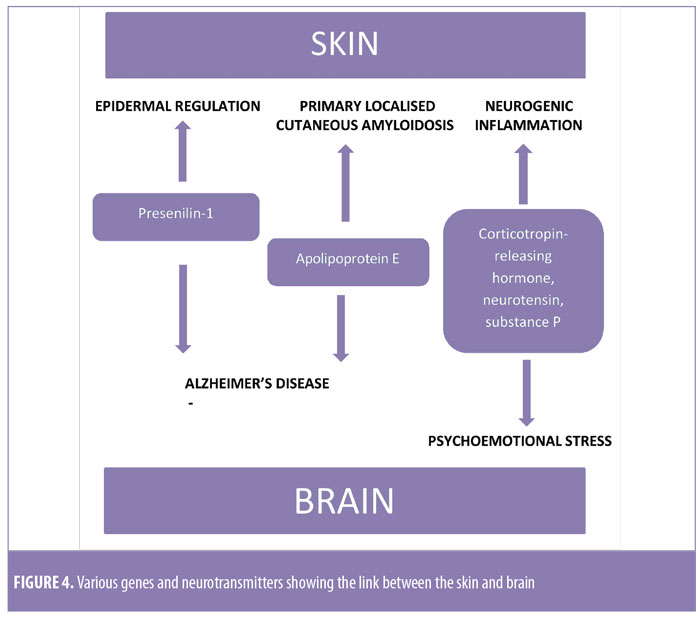
Schreml et al3 have shown in their study that genes such as presenilin-1 and ApoE4 (Figure 4) are involved in amyloidogenesis in the skin and brain. Presenilin-1 is an amyloid precursor that is involved in epidermal growth factor receptor turnover, and its loss is associated with cutaneous inflammatory diseases. Physiological changes in the skin of patients with Alzheimer’s disease have been reported. These alterations include lower oxidant defenses, dysfunctional bradykinin receptor signaling in fibroblasts, and the expression of ?-amyloid and tau proteins by mastocytes. Amyloid beta-protein precursor (AbPP) can be detected in the skin of Alzheimer’s disease patients, which acts as an autocrine growth stimulus in the epidermis and is released by keratinocytes. These findings support the close connection between skin and brain amyloidopathies. Other evidence supporting this skin-brain connection is the presence of psycho-emotional stress-induced neurogenic inflammation and pruritus. Pruritus can cause scratching, which can lead to inflammation. This inflammation can induce conformational changes in proteins, which can lead to amyloid deposition.3 Mediators that cause inflammation include corticotropin-releasing hormone (CRH), neurotensin, and substance P (Figure 4). Therefore, inflammatory cutaneous diseases might lead to amyloid deposition in the brain, while neurogenic inflammation might lead to skin amyloidosis. Amyloid deposition in the skin and brain is closely connected because of similar pathogenic mechanisms.
Conclusion
There is a paucity of data regarding the concurrent occurrence of skin and brain amyloidopathies. The correlation between the two organs is not well explored in the literature, and further study is necessary. The establishment of a link between the skin-brain axis will improve understanding of the role that anti-amyloid antibodies play in the treatment of cutaneous and neurodegenerative amyloidoses.
References
- Tillement JP, Lecanu L, Papadopoulos V. Amyloidosis and neurodegenerative diseases: current treatments and new pharmacological options. Pharmacology. 2010;85(1):1–17.
- Clos AL, Kayed R, Lasagna- Reeves CA. Association of skin with the pathogenesis and treatment of neurodegenerative amyloidosis. Front Neurol. 2012;20;3:5
- Schreml S, Kaiser E, Landthaler M, et al. Amyloid in skin and brain: What’s the link?. Exp Dermatol. 2010;19(11):953–957.
- Bandhlish A, Aggarwal A, Koranne RV. A clinico-epidemiological study of macular amyloidosis from north India. Indian J Dermatol. 2012;57(4):269–274.
- Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature. 1989; 341(6239):226–230.
- Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29(14):1924–1933.
- Murphy MP, Levine H 3rd. Alzheimer’s disease and beta-amyloid peptide. J Alzheimers Dis. 2010;19(1):311–323.
- Krishna A, Nath B, Dhir GG, et al. Study on epidemiology of cutaneous amyloidosis in northern India and effectiveness of dimethylsulphoxide in cutaneous amyloidosis. Indian Dermatol Online J. 2012;3(3):182–186.
- Jayabhanu AA, Bubna AK, Rangarajan S, et al. A clinicopathologic study of cutaneous amyloidosis at a tertiary health care center in South India. Pigment Int. 2016;3:17–23.

