 J Clin Aesthet Dermatol. 2018;11(8):15–18
J Clin Aesthet Dermatol. 2018;11(8):15–18
by Maria Azevedo, MD; Leonardo Bandeira, md; Cybelle Luza, MD; Alyne Lemos, MD; Francisco Bandeira, md, phd, face
Drs. Azvedo, Luza, and Lemos are with the Division of Endocrinology and Diabetes at Hospital Agamenon Magalhaes and the University of Pernambuco Medical School in Recife, Brazil. Drs. L. Bandeira and F. Bandeira are with the University of Pernambuco Medical School and the FBandeira Endocrine Institute in Recife, Brazil.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective. We sought to evaluate serum 25-hydroxycholecalciferol (vitamin D [25-OHD]) levels, skin phototype, and sun index in a sample of patients to determine the association between these factors and metabolic risk.
Design. This was a cross-sectional study involving 729 adults (50.2% male). Mean age was 65.13±9.18 years, sun index 5.71±5.06, body mass index (BMI) 27.60±5.34 kg/m2, and waist circumference 97.29±12.08cm. Hypertension, metabolic syndrome, and Type 2 diabetes were reported in 77.8, 74.5, and 38.9 percent, respectively; Fitzpatrick Skin Types III and IV were reported in 60.6 percent.
Results. Mean serum 25-OHD was 25.72±10.91ng/mL; 31 percent of subjects had serum 25-OHD below 20ng/mL, and 63.1 percent had serum 25-OHD below 30ng/mL. Although there were no significant differences between the vitamin D deficient and sufficient groups regarding age, BMI, waist circumference, or presence of diabetes, in the group with 25-OHD less than 20ng/mL (sun index of 4.5±4.08), higher serum triglycerides and lower high-density lipoprotein cholesterol (HDL-C) levels were measured: triglycerides 179.14±103.53 versus 161.63±90.23mg/dL (p=0.029) and HDL-C 43.48±12.38 versus 45.94±14.14mg/dL (p=0.018) compared to the group with 25-OHD levels of 20ng/mL or higher (sun index: 6.25±5.36). Considering less than 25th percentile (25-OHD: 18.7ng/mL) and 75th percentile or higher (25-OHD: 30.8 ng/mL), the differences in serum triglycerides remained significant: 176.63±103.79 versus 157.47±80.49 (p=0.039).
Conclusion. We found a high prevalence of vitamin D deficiency in individuals with high sun exposure, regardless of age, BMI, and waist circumference. This deficiency was associated with increased serum triglycerides and decreased HDL-C levels.
Keywords: Vitamin D, UV exposure, skin phototype, metabolic risk, cardiovascular risk
Introduction
Studies have shown a high prevalence of vitamin D deficiency among different ethnic groups, geographic regions, and age groups.1 With the identification of vitamin D nuclear receptors in various organs, such as the pancreas, breast, prostate, brain, heart, and immune system, it has become apparent that vitamin D deficiency is associated with several disorders, including metabolic syndrome (MS) and insulin resistance.2
The 1?-hydroxylase enzyme responsible for the synthesis of the active form of vitamin D is also present in various cells, such as the pancreatic ?-cell, acting in the insulin gene promoter,3 independent of parathyroid hormone (PTH), and enabling the antiproliferative, immunomodulatory, and pro-differentiating effects of vitamin D.4,5 It has been suggested that vitamin D increases insulin sensitivity by stimulating the expression of insulin receptor (INS-R) by indirect action, regulating the influx of calcium through the membrane cell required for insulin activity and its modulatory effect on the formation and action of cytokines3 (inactivation of nuclear factor kappa beta), increasing the survival of beta cells. Vitamin D is also involved in the regulation of angiotensin II, inhibiting insulin action in skeletal muscles and vessels.6,7
With a growing prevalence of metabolic syndrome among the general population, it is crucial to identify new modifiable risk factors, such as vitamin D deficiency. When added to a balanced diet and regular physical activities, vitamin D supplementation has the potential to assist in health management and prevention of disease.2
Even in areas with high sun exposure, high prevalence of vitamin D deficiency has been reported, with sun exposure alone being insufficient to achieve adequate amounts of vitamin D in most individuals.8 This study sought to determine the prevalence of vitamin D deficiency and its association with metabolic risk in individuals with high levels of sun exposure.
Methods
Patients who attended our primary care and endocrinology clinics during a six-month period (August 2013–February 2014) were consecutively enrolled in the study (N=729, age 33–92 years). Exclusion criteria included chronic use of glucocorticoids, malignancy in the last five years (except basal cell carcinoma), malabsorption syndrome, use of anticonvulsants or lithium, severe hepatic and/or renal disease, and metabolic bone diseases (except for osteopenia and osteoporosis). All patients included in the study provided informed consent, and the study was approved by the Ethics in Research Committee of Agamenon Magalhães Hospital.
Sun index was calculated by the number of hours per week of sun exposure multiplied by the fraction of the total body surface area exposed.10 For the skin phototypes, the Fitzpatrick classification, which indicates the tolerance of the skin to ultraviolet light based on the account of the individual, sunburn, and tanning capacity, was used.11 The Fitzpatrick skin types are as follows: Type I=very fair skin, always burns, never tans; Type II=clear skin, always burns and sometimes tans; Type III= less clear skin, sometimes burns and always tans; Type IV= light brown skin, rarely burns and always tans; Type V= dark brown skin, never burns and always tans; and Type VI= black skin, never burns and always tans. Body mass index (BMI) was calculated according to guidelines set forth by the World Health Organization.12 Waist circumference (WC) was measured according to the criteria of the International Diabetes Federation (IDF) and the criteria of the National Cholesterol Education Program (NCEP) Third Report.13,14 Triglycerides and total and high-density lipoprotein (HDL) cholesterol levels were measured using an auto-analyzer (Vitros system, Ortho Clinical Diagnostics, Johnson & Johnson, Raritan, New Jersey). Serum 25-hydroxycholecalciferol (vitamin D [25-OHD]) was measured by electrochemiluminescent competitive immunoassay (Liaison®, DiaSorin, Stillwater, Minnesota), and patients were grouped according to a cutoff point of 25-OHD level less than 20ng/mL or according to the lower compared to the upper quartile.15,16
Statistical analysis. The results were analyzed using the following measures of descriptive statistics: minimum and maximum values, mean, median, standard calculated deviation, and percentages. The Student’s t-test was used to compare two categories of variables. The confidence interval was 95 percent, and the significance level was less than five percent. The statistical program used for test decision was SPSS Version 17 and STAT Version 11.
Results
Baseline characteristics of the study population are shown in Table 1.
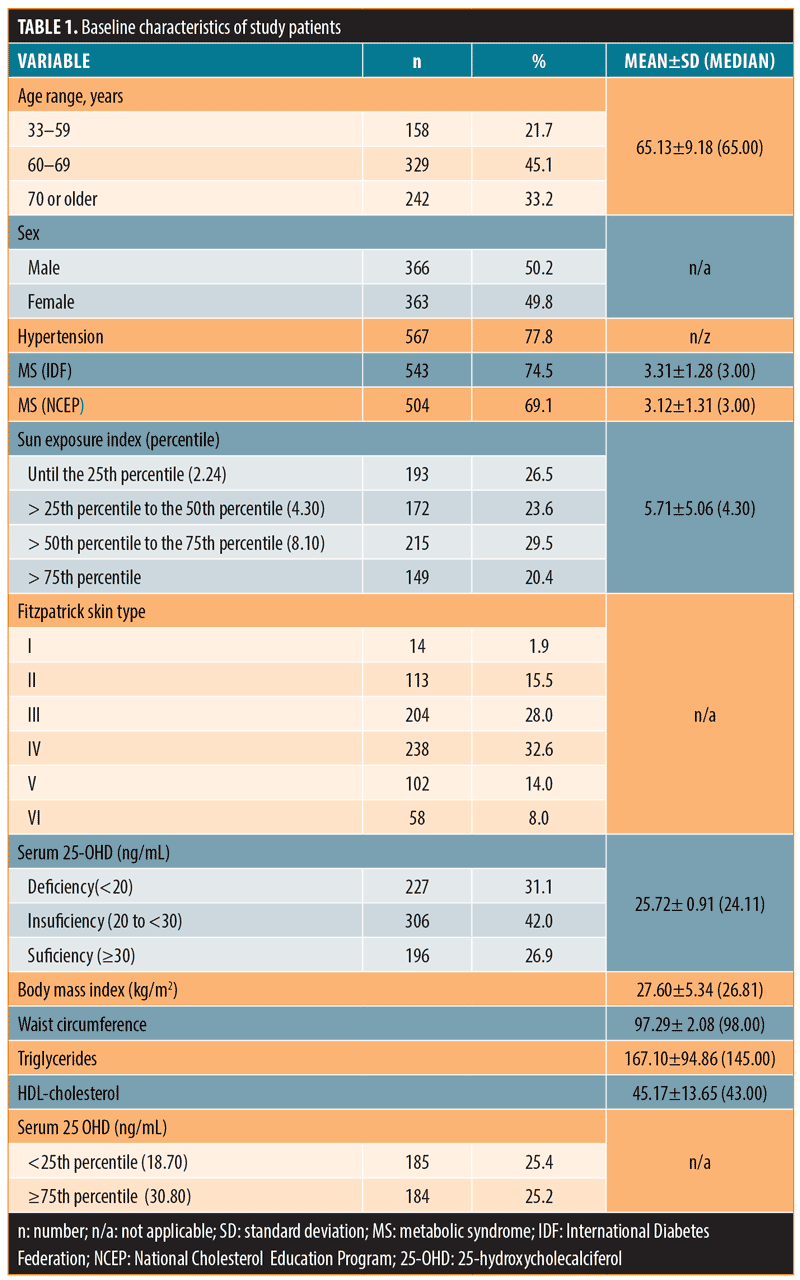
We studied 729 subjects (50.2% male) with a mean age of 65.13±9.18 years, sun index 5.71±5.06, BMI 27.60±5.34kg/m2, and waist circumference 97.29±12.08cm. Hypertension, metabolic syndrome, and Type 2 diabetes were reported in subjects at rates of 77.8, 74.5, and 38.9 percent, respectively; Fitzpatrick Skin Types III and IV were reported in 60.6 percent of subjects. Mean serum 25-OHD was 25.72±10.91ng/mL; 31 percent of subjects had serum 25-OHD below 20ng/mL, and 63.1 percent had serum 25-OHD below 30ng/mL.
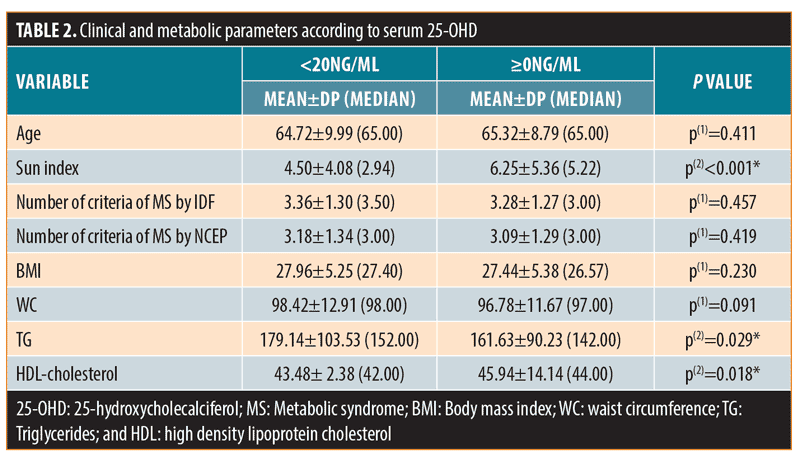
Compared to the group with serum 25-OHD at or above 20ng/mL, the group with serum 25-OHD less than 20ng/mL (sun index of 4.5±4.08) showed higher serum triglycerides (179.14±103.53mg/dL [p=0.029]) and lower HDL-C (43.48±12.38mg/dL [p=0.018]), despite no significant differences between the two groups regarding age, BMI, WC, and the number of MS symptoms (Table 2).
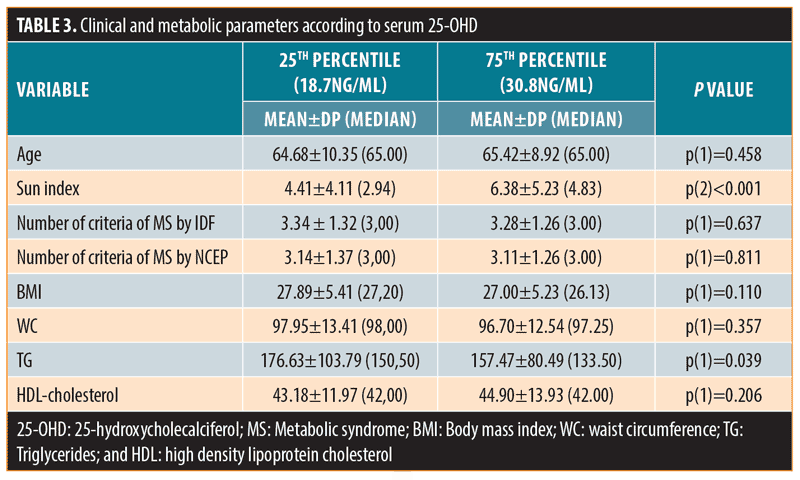
When subjects were grouped according to serum 25-OHD below the 25th percentile (25-OHD: 18.7ng/mL) or above the 75th percentile (25-OHD: 30.8ng/mL), the differences in serum triglycerides remained significant: 176.63±103.79 or 157.47±80.49 (p=0.039), respectively (Table 3).
Discussion
In the present study, we observed a high prevalence of vitamin D deficiency (25-OHD levels <20ng/mL) and vitamin D insufficiency (25-OHD levels <30ng/mL) at rates of 31.1 and 73.1 percent, respectively. Only 26.9 percent of subjects had sufficient levels of vitamin D (25-OHD ?30ng/mL, according to Endocrine Society guidelines16). Considering the latitude where the subjects live (8°S), this is in agreement with other studies from our institution involving a smaller number of subjects. Bandeira et al17 evaluated 99 postmenopausal women attending an endocrine clinic and observed that 43.7 percent had 25-OHD levels below 25ng/mL (mean 25-OHD serum was 28.7ng/mL). In addition, Cabral et al18 conducted a study in Recife that included 284 elderly men who were attending a primary care clinic, and reported the prevalence of vitamin D deficiency (25-OHD levels <20ng/mL) at 31.5 percent and insufficiency (25-OHD levels <30ng/mL) at 66.7 percent. These results suggest that even in low latitudes with abundant sunshine throughout the year where individuals receive high sun exposure in daily life, most individuals remain below optimal concentrations serum 25-OHD levels.
Rajakumar et al19 conducted a study of 237 adolescents (mean age 12.7 years) living in Pennsylvania (latitude 40°N), and observed a mean 25-OHD level of 19.4ng/mL in 73 percent of African-American teens and serum 25-OHD levels below 20ng/mL in 40 percent of Caucasian teens. There were negative associations between serum 25-OHD and BMI and percentage of total body fat and a positive association with HDL cholesterol.
Regarding the presence of Type 2 diabetes as part of the metabolic risk profile, the Third National Health and Nutrition Examination Survey (NHANES III) showed an inverse association between serum 25-OHD levels and cardiovascular disease among patients with Type 2 diabetes.20 In a case-control study with 608 women with Type 2 diabetes and 559 controls recruited from the Nurses Health Study, Pittas et al21 observed an inverse relationship between Type 2 diabetes and serum 25-OHD levels (48% lower incidence in women in the highest quartile of 25-OHD) independent of conventional risk factors. Similarly, in a prospective study that followed 524 individuals over 10 years, Forouhi et al22 observed an inverse association between 25-OHD and risk of hyperglycemia, insulin resistance, and metabolic syndrome.
Considering this research, it is not yet clear whether the metabolic alterations associated with vitamin D deficiency can be reversed by sun exposure and/or adequate vitamin D supplementation. To illustrate, in a clinical trial conducted by Wood et al,23 305 postmenopausal women aged 60 to 70 years were randomized to receive vitamin D3 400IU, 1,000IU, or placebo. None of the women showed significant differences in serum triglycerides, LDL cholesterol, HDL cholesterol, apoprotein A and B, HOMA-IR, c-reactive protein, or serum IL-6 following vitamin D3 supplementation. Thus, it is yet to be determined if supplementation with higher vitamin D3 doses with or without responsible sun exposure can have beneficial effects on cardiovascular risk factors.24 Ju et al25 conducted a meta-analysis of cross-sectional and longitudinal studies from various countries at different latitudes and reported significant inverse associations between serum 25-OHD levels and the presence of metabolic syndrome, although the cut points used were extremely variable (from <11.5ng/mL to 23ng/mL to <31ng/mL to 41ng/mL). This is in agreement with our data, which, by latitude, demonstrated an average serum 25-OHD of 25.7ng/mL, and that suggest that even in populations with a high rate of sun exposure, higher values of serum 25-OHD would be more protective.
The importance of this study lies in the assertion that there are still little data showing the association between sun exposure, skin phototype, vitamin D deficiency, and metabolic risk, especially in areas with abundant ultraviolet (UV) radiation. In this regard, it has yet to be elucidated whether the skin of individuals exposed to high intensity sunlight in daily life, as seen in our study, would exhibit photoprotective mechanisms beyond tanning.26,27 For example, the cholesterol side chain cleavage enzyme CYP11A1 is expressed in skin and produces 20-hydroxyvitamin D3 (20OHD). Using contact hypersensitivity, which is suppressed by UV irradiation, topical application of 20OHD to mice protected them against UV-induced immunosuppression.26
On the other hand, some genetic variants can lead to different responses of serum 25-OHD to provitamin D.27 Minor aleles of CYP2R1 (which belongs to cytochrome P450 superfamily IIR1 and encodes hepatic 25-hydroxylase), such as rs10500804, and group-specific complement (which encodes VDR and DBP), such as rs4588 and rs7041, are associated with a reduced response of serum 25-OHD to UV radiation. Consequently, poorer efficacy of sun exposure for the prevention and treatment of vitamin D deficiency can result.
Conclusion
We found a high prevalence of vitamin D deficiency in our study subjects. This deficiency was associated with higher serum triglycerides and lower levels of serum HDL-C concentrations in individuals with very high rates of sun exposure in daily life, independent of age, BMI, or waist circumference. Taken together, these data not only suggest a direct association between vitamin D deficiency and metabolic risk, but suggest a lack of effectiveness that frequent sun exposure might have as a measure to prevent vitamin D deficiency, especially in tropical areas.
References
- Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner.1998;13:325–349.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371.
- Bikle D. Nonclassic Actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34.
- Bland R, Markovic D, Hills CE, et al. Expression of 25 hydroxyvitamin D3 -1?-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89:121–125.
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511.
- Sowers JR. Insulin resistance and hypertension. J Physiol Heart Circ Physiol. 2004;286(5): H1597–1602.
- Wei Y, Sowers JR, Clark SE, et al. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappa B activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294(2):E345–351.
- Correia A, Azevedo M, Gondim F, Bandeira F. Ethnic aspects of vitamin D deficiency. Arq Bras Endocrinol Metab. 2014;58(5):540–544.
- Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metab. 2006;50(4):640–646.
- Barger-Lux MJ, Heaney RP. Summer sun exposure and 25 hydroxyvitamin D. J Clin Endocrinol Metab. 2002;87(11):4952–4956.
- Fitzpatrick T. B. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871.
- Organização Mundial de Saúde (OMS). Obesity: preventing and managing the global epidemic. Report of a World Health Organization Consultation. 2000. (WHO Obesity Technical Report Series, n. 284).
- Alberti KG, Zimmet P, Shaw J. Epidemiology Task Force Consensus Group: the metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062.
- Executive summary of the third report of the National Cholesterol Program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adults Treatment Panel III). JAMA. 2001;285(19):2486–2497.
- Holick MF. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline: J Clin Endocrinol Metab. 2011;96:1911–1930.
- Rosen CJ, Abrams SA, Aloia JF, et al. IOM (Institute of medicine) committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab. 2012;94:1146–1152.
- Bandeira F, Griz L, Freese E, et al. Vitamin D deficiency and its relationship with bone mineral density among postmenopausal women living in the tropics. Arq Bras Endocrinol Metab. 2010;54(2): 227–232.
- Cabral MA, Borges CN, Maia JM, et al. Prevalence of vitamin D deficiency during the summer and its relationship with sun exposure and skin phototype in elderly men living in the tropics. Clin Interv Aging. 2013;8:1347–51.
- Rajakumar K, de las Heras J, Chen TC, et al. Vitamin D status, adiposity, and lipids in black american and caucasian children. J Clin Endocrinol Metab. 2011;96(5):1560–1567.
- Stragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D3, diabetes and ethnicity in the National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818.
- Pittas AG, Sun Q, Manson JE, et al. Plasma 25-hydroxyvitamin D concentration and risk of incident Type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023.
- Forouhi NG, Luan J, Cooper A, et al. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008; 57:2619–2625.
- Wood AD, Secombes KR, Thies F, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled randomized, controlled trial. J Clin Endocrinol Metab. 2012;97(10):3557–3568.
- Hartley M, Hoare S, Lithander FE, et al. Comparing the effects of sun exposure and vitamin D supplementation on vitamin D insufficiency, and immune and cardiometabolic function: the Sun Exposure and Vitamin D Supplementation (SEDS) Study. BMC Public Health. 2015;15:115.
- Ju SY, Jeong HS, Kim do H. Blood vitamin D status and metabolic syndrome in the general adult population: a dose-response meta-analysis. J Clin Endocrinol Metab. 2014;99: 1053–1063.
- Tongkao-on W, Carter S, Reeve VE, et al. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2014; doi.org/10.1016/j.jsbmb.2014.11.015
- Petersen RA, Larsen LH, Damsgaard CT, et al. Common genetic variants are associated with lower serum 25-hydroxyvitamin D concentrations across the year among children at northern latitudes. Br J Nutr. 2017; doi:10.1017/S0007114517000538

