J Clin Aesthet Dermatol. 2019;12(6):30–41
 by James Q. Del Rosso, DO; Theodore Rosen, MD; Dimitry Palceski, DO; and Maria Jose Rueda, MD
by James Q. Del Rosso, DO; Theodore Rosen, MD; Dimitry Palceski, DO; and Maria Jose Rueda, MD
Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada. Dr. Rosen is with Baylor College of Medicine in Houston, Texas. Dr. Palceski is with the Reflections Dermatology & Center for Skin Care in Orlando, Florida. Dr. Rueda is with Galderma Laboratories, LP in Fort Worth, Texas.
FUNDING: This study was funded by Galderma Laboratories, LP.
DISCLOSURES: Drs. Del Rosso, Rosen, and Palceski are consultants and investigators for Galderma. Dr. Del Rosso has served as a research investigator and speaker for Galderma. Dr. Rueda was an employee of Galderma at the time this article was written.
ABSTRACT: Background. Antibiotic resistance presents a threat to public health. In dermatology, antibiotics are used extensively for the treatment of acne, sometimes for extended periods. Thus, awareness of antibiotic resistance among dermatology patients is relevant in clinical practice.
Methods. An online survey assessed antibiotic resistance awareness in adults with acne (n=809) and the parents of adolescents with acne (n=210).
Results. More than 80 percent of subjects said that they were “somewhat familiar” or “very familiar” with antibiotic resistance. Overall, 86 percent of the survey respondents identified the correct definition of antibiotic resistance, with parents more likely than their children to choose the proper definition of resistance, as follows: “When antibiotics and/or antibacterials are used for a period of time, the infectious organism adapts to them and becomes immune, resulting in less effective treatment” (95% confidence interval). Among subjects who might have been prescribed antibiotic treatment for their acne, including individuals that reported antibiotic treatment and individuals that were not sure, 76.9 percent reported that they would be very or extremely likely to use effective antibiotic-free options if given the opportunity. More than 90 percent of people with acne and their parents agreed that healthcare providers should do more to educate patients about antibiotics and antibiotic resistance.
Conclusions. This survey indicated that patients with acne and their parents think more should be done to educate the public about about the potential risks associated with antibiotic use and the availability of antibiotic-free treatment options. Discussions with patients about antibiotic therapies, antibiotic resistance, and alternative therapies represent areas of opportunity for healthcare providers in dermatology.
KEYWORDS: Acne, antibiotic resistance, antibiotics
Antibiotic resistance is an emerging threat to public health, a concern that has recently been highlighted in the medical and general public literature.1–3 Despite the widespread interest in the topic of antibiotic stewardship, limited data are available describing public awareness and understanding of the issues surrounding antibiotic resistance. Dermatology patients are often prescribed antibiotic therapies, both for short-term infections and for chronic inflammatory dermatoses.4 Patients with acne and rosacea, together making up approximately 20 percent of the United States (US) population, are frequently offered or prescribed antibiotic therapies.5 These conditions are often treated with tetracycline-class antibiotics, or, in the case of acne, topical clindamycin, both of which are also used systemically in the treatment of methicillin-resistant Staphylococcus aureus (MRSA).4,6,7 More generally, in 2013, in the US alone, dermatological diseases accounted for 5.8 million oral antibiotic prescriptions and 3.5 million topical antibiotic prescriptions.8 Because the number of dermatology patients exposed to antibiotic therapies is so extensive, they represent an excellent subpopulation in which to study antibiotic resistance awareness.
With at least 50 million people with acne living in the US, acne vulgaris (AV) is one of the most common skin diseases in the country.9–11 Though AV is most prevalent among adolescents, it can occur at any age, often starting in the preteen years and persisting into adulthood.11,12 AV is an inflammatory cutaneous disorder that can affect the face, back, and/or chest, presenting with any combination of comedones (open or closed), papules, pustules, and/or nodules.9 The traditional understanding of acne pathophysiology has included four primary components: : abnormal keratinization and follicular desquamation, changes in sebum composition and/or production, proliferation of Cutibacterium acnes (C. acnes, formerly called Propionibacterium acnes), and multiple pathways of inflammation.13,14 Additionally, hormonal factors (such as increased androgens or corticotropin-releasing hormone) can contribute to acne pathophysiology by promoting alterations in the composition and quantity of sebum.15–17 Advances in the understanding of acne pathophysiology have revealed inflammation to be a common contributor across all forms of acne.13,14,18–22 While the contribution of C. acnes to acne pathology seemingly justifies the widespread use of antibiotic treatment, the precise role of C. acnes in acne pathogenesis remains unclear.23,24 Some strains of C. acnes are proinflammatory and might activate receptors of the innate immune system, which, in turn, lead to the activation of inflammatory cytokine cascades.19–21 Furthermore, increased antimicrobial peptide levels have been observed in AV, which might be the result of C. acnes proliferation or C. acnes-induced changes in sebum levels and composition.25–27 Alterations in sebum and C. acnes within the pilosebaceous unit also appear to contribute to hyperkeratinization and comedone formation.14 However, despite the apparent contribution of C. acnes to AV, the bacterium is commensal, with proliferation of proinflammatory strains contributing to host response patterns that take part in the development of AV lesions. AV is not generally considered communicable.14,28–31
Therapies used by clinicians to initiate treatment of AV include topical retinoids (e.g., adapalene, tretinoin, tazarotene) and antimicrobial agents (e.g., benzoyl peroxide or topical/oral antibiotics); the selection of agents is correlated with the severity of AV, often with a combination therapy approach.9,10,14,32 Fixed combination topical treatments provide convenience, with efficacy and safety established in clinical studies and with real-world use.33–38 Additionally, therapies for adult women with AV can include combination oral contraceptives and oral spironolactone.9,14,32,39 With increasing severity, recommendations from the Global Alliance and American Academy of Dermatology include the addition of oral antibiotics or oral isotretinoin; however, the use of topical and oral antibiotics in acne therapy has become more scrutinized in recent years due to global concerns regarding antibiotic resistance.4,6,7,9,10,40–42 Dermatologists prescribe a relatively higher percentage of antibiotics (3–5% of all antibiotics prescribed across specialties) compared to other types of physicians, with up to two-thirds of these prescriptions being written for AV and, therefore, presumably requiring an extended course of therapy.4,8 Because the length of exposure to antibiotics is one of the important contributing factors in the emergence of antibiotic resistance among exposed bacteria, the judicious use of such agents in dermatology is one of many steps that can be taken to mitigate the development of antibiotic-resistant organisms.4,6,7,41–43
The progressive emergence of bacterial resistance is one potential consequence of widespread clinical antibiotic use. While prophylactic antibiotic use in livestock is considered to be a major factor contributing to the rise in microbial resistance, clinical antibiotic use also contributes to an environment fostering the development of resistance.1,4,6,44 Additionally, bacterial resistance is not the only inevitable consequence associated with the clinical use of antibiotics. Both topical and oral antibiotics have been associated with changes in the human microbiome (the vast array of commensal, symbiotic, opportunistic, and pathogenic microorganisms that reside on and within the human body).41,45 Topical antibiotics alter the cutaneous microbiota, leading to resistance patterns, even at anatomical sites distal to the site of application. For example, alterations in bacterial flora within the anterior nares and at remote cutaneous sites have been observed following facial application of antibiotics.46,47 Likewise, oral antibiotics can lead to changes in the gastrointestinal (GI) microbiome. This can lead to alterations in commensal competition and flora balance, as well as to the creation of reservoirs of antibiotic resistance within specific microbiota. Systemic antibiotic treatment can also result in opportunistic infection by Clostridium difficile (C. difficile) and contribute to GI-related adverse events.9,43,45
The impact of antibiotic use and misuse on the emergence of antibiotic resistance, both in the US and worldwide, has led the US Centers for Disease Control and Prevention (CDC) to focus on educational and public awareness measures to support antibiotic stewardship. Among the resistant organisms that can result from antibiotic therapy, carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae (N. gonorrhoeae) (over half of which display resistance to tetracycline), multidrug-resistant Pseudomonas aeruginosa, MRSA, and C. difficile have been identified as urgent threats by the CDC.48 Worldwide, many common bacteria, such as Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae), are exhibiting high rates of resistance, negatively impacting available treatments for common infections, including urinary tract infections and pneumonia.49
The current study measured the attitudes toward and awareness of antibiotic resistance among adults with AV and the parents of children/adolescents with AV. Among respondents, this study assessed their experience with antibiotics and antibiotic-free acne treatments, awareness of and experience with superbugs (i.e., strains of bacteria resistant to antibiotics), and agreement with potential actions to limit the future impact of antibiotic resistance.
Methods
Subjects. The study included survey data from 1,019 subjects. Respondents belonged to one of two groups: 1) People aged 17 to 40 years who had received a prescription treatment (oral or topical) for AV in the previous year (n=809); and 2) parents of children or adolescents (aged 9–17 years) who had received a prescription for AV in the past year (n=210). All subjects were US residents. Participants were required to agree to release contact information in the case of adverse events and/or product-quality complaints. Respondents were obtained from a propriety American Consumer Opinion Panel (Decision Analyst, Arlington, Texas). From the panel, 183,037 panelists were invited to undergo screening, 14,224 completed the screening, and 1,019 qualified and completed the full survey. Patients who met inclusion criteria were informed that individual responses would be anonymous and confidential and were invited to complete the survey by clicking continue.
Survey design. Subjects were surveyed online through Decision Analyst, and included a nationally representative (US, including Alaska and Hawaii) sample of patients with AV, as well as parents of adolescent patients with AV. The survey sections utilized in this study included three main categories of questioning:
Demographic questions (employment status, marital status, household income, ethnic background, and educational level; five questions)
Study eligibility determination (age, sex, recent diagnoses, and treatments prescribed; 12 questions, some with multiple parts)
Assessment of awareness of antibiotic resistance and superbugs, experiences with antibiotic and antibiotic-free therapies for AV, emotional impact of AV, and agreement with potential actions to increase awareness and limit the impact of antibiotic resistance (29 questions, some with multiple parts).
The survey included a combination of free-response and aided (prompted, recognition-based) response questions. For the multipart questions, depending on responses, some respondents were exempted from subsequent nonapplicable questions. Survey results were descriptively tabulated and 90 and 95 percent confidence intervals (CIs) were used to identify significant differences between the groups.
Results
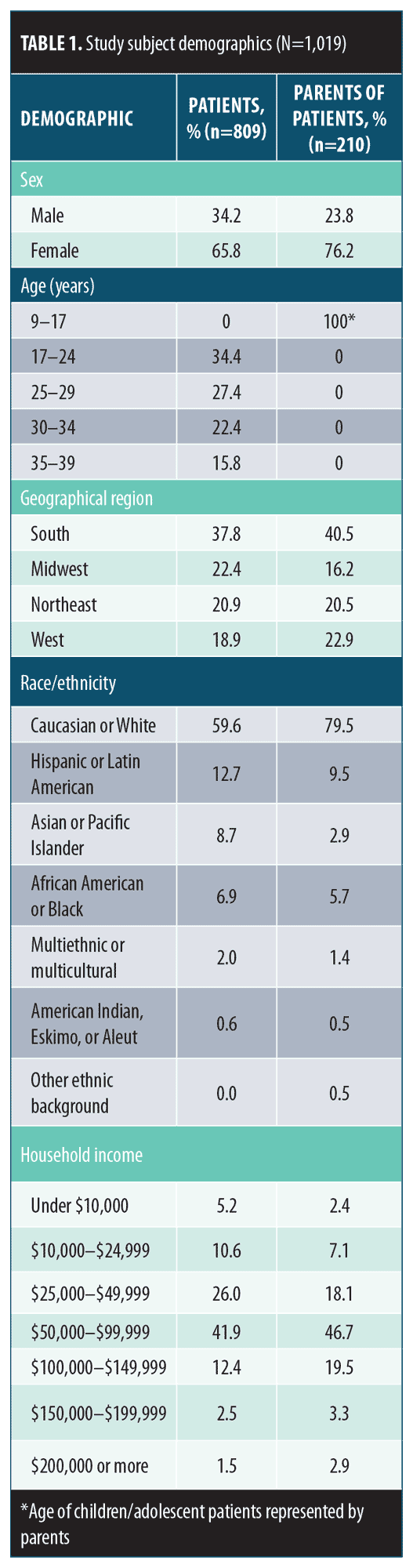 Subject demographics. Subject demographics are presented in Table 1. The majority of respondents were women (65.8% of adult patient respondents and 76.2% of parent respondents) and identified as Caucasian or white (59.6% of adult patients and 79.5% of parents). The mean household income of adult patient respondents was $65,200, and the mean household income of parent respondents was $78,900. Respondents resided throughout the US.
Subject demographics. Subject demographics are presented in Table 1. The majority of respondents were women (65.8% of adult patient respondents and 76.2% of parent respondents) and identified as Caucasian or white (59.6% of adult patients and 79.5% of parents). The mean household income of adult patient respondents was $65,200, and the mean household income of parent respondents was $78,900. Respondents resided throughout the US.
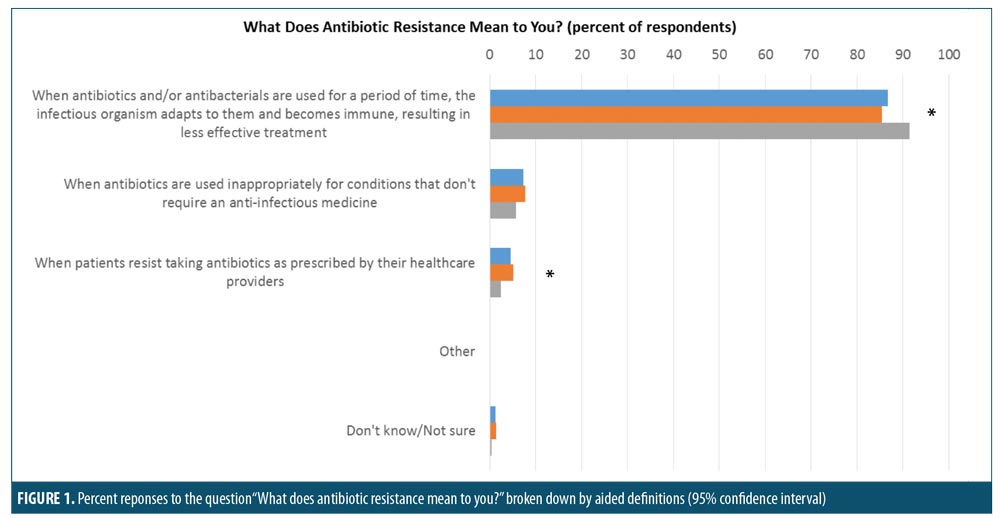
Respondent understanding of antibiotic overuse and efficacy. The majority of respondents had a basic understanding of antibiotic resistance, with parents more likely than adult patients to correctly define antibiotic resistance as “when antibiotics and/or antibacterials are used for a period of time, the infectious organism adapts to them and becomes immune, resulting in less effective treatment.” This response was chosen by 91.4 percent of parents versus 85.4 percent of adult patients (significantly different at 95% confidence interval (CI); Figure 1).
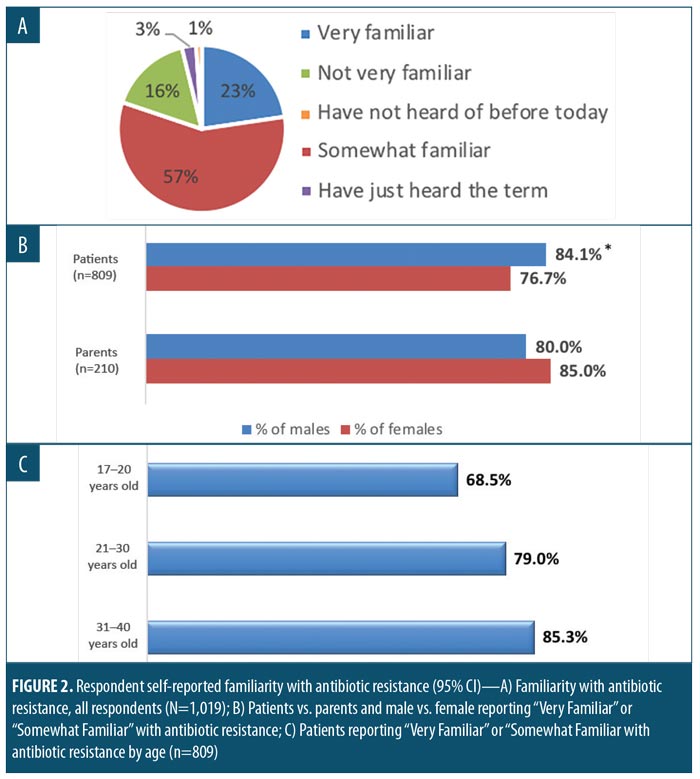
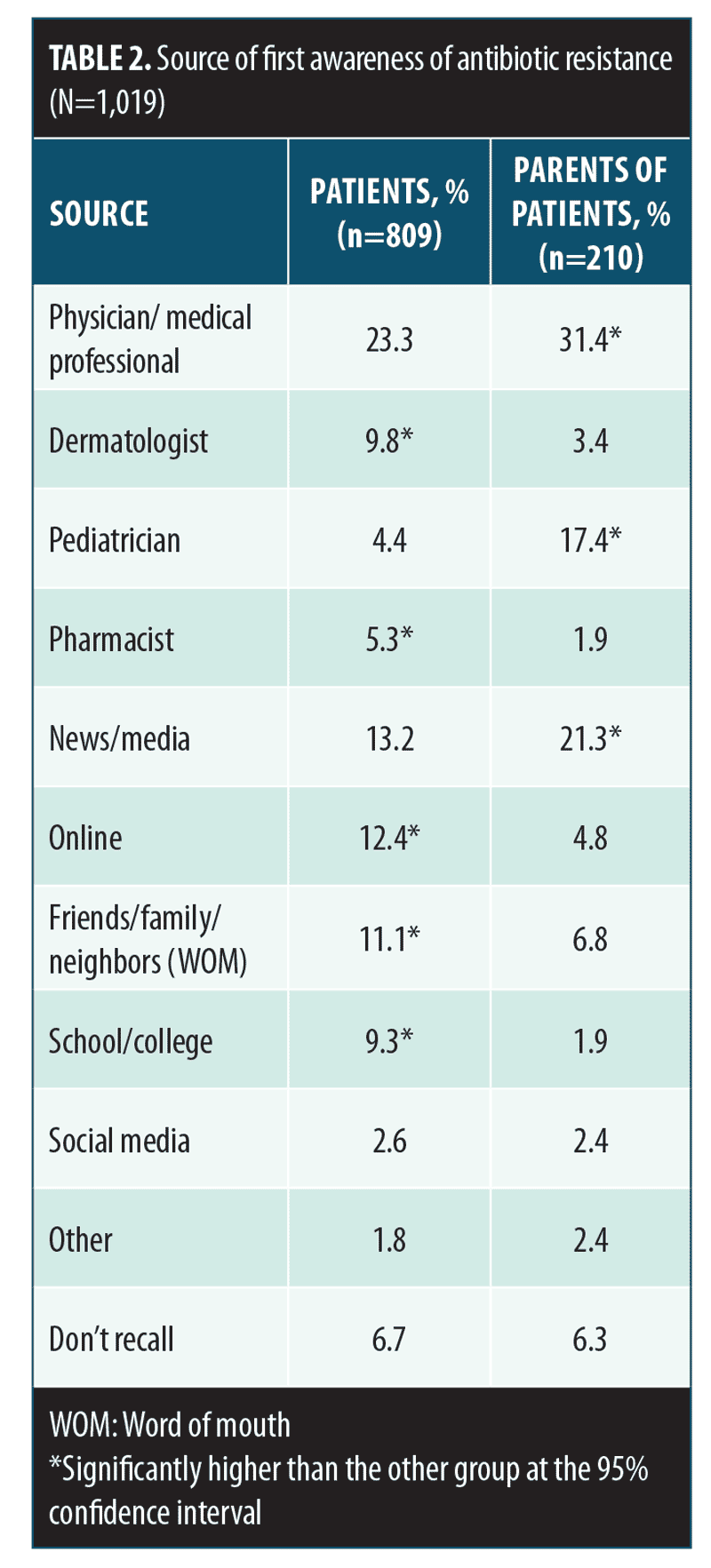
More than 80 percent of respondents said that they were at least somewhat familiar with the topic of antibiotic resistance (Figure 2A). Antibiotic resistance awareness was high among both sexes, although some differences and trends were seen in response rates. A greater percentage of male patients with AV, compared to female patients (significant at 95% CI), and a greater percentage of female parents, compared to male parents (not significant [NS]), indicated that they were very or somewhat familiar with antibiotic resistance (Figure 2B). Antibiotic resistance awareness was high among all age groups, with self-reported awareness among patients with AV increasing with age (Figure 2C). Among both patients and parents, the most frequently reported first source of antibiotic resistance information was a physician or medical professional. In contrast, less common sources of “first awareness,” including the news media or online sources, were reported at different rates between the two groups of respondents (Table 2).
Familiarity and concerns around oral versus topical antibiotics. Overall familiarity with the risk of antibiotic resistance resulting from the use oral antibiotics was high. More than 84 percent of respondents agreed that one can develop antibiotic resistance from oral antibiotics. Agreement was similar among patients (83.8%) and parents (86.5%). Agreement that one can develop antibiotic resistance from topical antibiotics was lower, at an average of 62.1 percent of patients (62.7% patients, 59.9% parents). There was a significant difference (at the 95% CI) in respondent knowledge concerning the consequences of antibiotic overuse when patients and parents were compared: 36.7 percent of patients versus 21.4 percent of parents responded that they “did not know overuse of antibiotics could increase (my) risk for an antibiotic-resistant infection.” Men (both patients and parents) were more likely to agree with this statement than women (significantly different at 95% CI).
The majority (56.2%) of respondents somewhat or completely agreed with the assertion that oral antibiotics are more likely to cause antibiotic resistance when compared to topical antibiotics used for the treatment of skin disorders. Only 31.2 percent of respondents agreed with the opposing statement, that topical antibiotics are more likely to cause antibiotic resistance when compared to oral antibiotics used for the treatment of skin conditions such as AV and rosacea.
 Perceptions of antibiotic efficacy in acne. A very small percentage of respondents (5.4%) agreed with the assertion that AV can only be treated by antibiotics, with a significantly greater percent of patients than parents thinking this to be true (6.1% vs. 2.9%, respectively, significant at 95% CI). Interestingly and surprisingly, the majority (74.2%) of respondents somewhat or completely agreed with the statement: “for acne, I would prefer to use (or have my children use) an effective antibiotic-free prescription treatment instead of a full-dose antibiotic.”
Perceptions of antibiotic efficacy in acne. A very small percentage of respondents (5.4%) agreed with the assertion that AV can only be treated by antibiotics, with a significantly greater percent of patients than parents thinking this to be true (6.1% vs. 2.9%, respectively, significant at 95% CI). Interestingly and surprisingly, the majority (74.2%) of respondents somewhat or completely agreed with the statement: “for acne, I would prefer to use (or have my children use) an effective antibiotic-free prescription treatment instead of a full-dose antibiotic.”
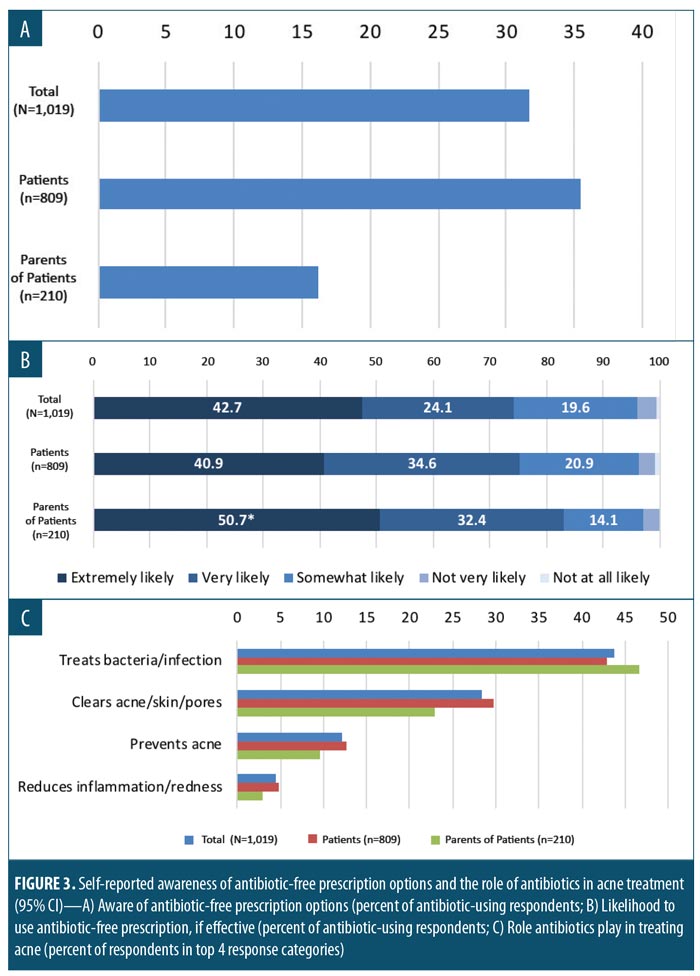
More than half (53.9%) of study subjects responded that they (or their children) were prescribed an antibiotic for AV during (approximately) the past year. Only 31.7 percent of antibiotics users (or those who were not sure whether they were prescribed an antibiotic) were aware of antibiotic-free prescription options. There was less awareness of antibiotic-free treatment options among parents than among patients (16.2% vs. 35.4%, respectively; Figure 3A). Among this population, 76.9 percent (75.4% patients, 83.1% parents, significantly different at 95% CI) said they would be very or extremely likely to use an antibiotic-free prescription treatment for AV if they were aware of the existence of effective, antibiotic-free therapeutic options (Figure 3B).
In the open-ended response to “what specific role do antibiotics play in treating acne?”, the greatest proportion of responses (43.7%) were associated with the role of antibiotics in the treatment of bacteria or infection (Figure 3C). In contrast, a minority of respondents (18.4%) indicated a belief that AV is an infectious disease, with this belief significantly more prevalent among patients than among parent respondents (19.9% vs. 12.9%, respectively, significant at 95% CI). An additional 17.2 percent of subjects indicated they were undecided concerning the infectiousness of AV.
Among antibiotic users, the primary reason reported for choosing this treatment option was that the respondents felt it would be more effective than antibiotic-free prescription treatment options (43%). Other top responses included treatment duration (23.9%) and a lack of awareness of antibiotic-free prescription treatment options (22.8%). Among subjects who were prescribed antibiotic-free treatments, the top reasons reported for the use of an antibiotic-free treatment option were: 1) “felt it would be just as/more effective than antibiotics” (43.3%); 2) “concerns about potential risk of antibiotic resistance” (26.9%); and 3) “relative ease of treatment/application” (26.5%).
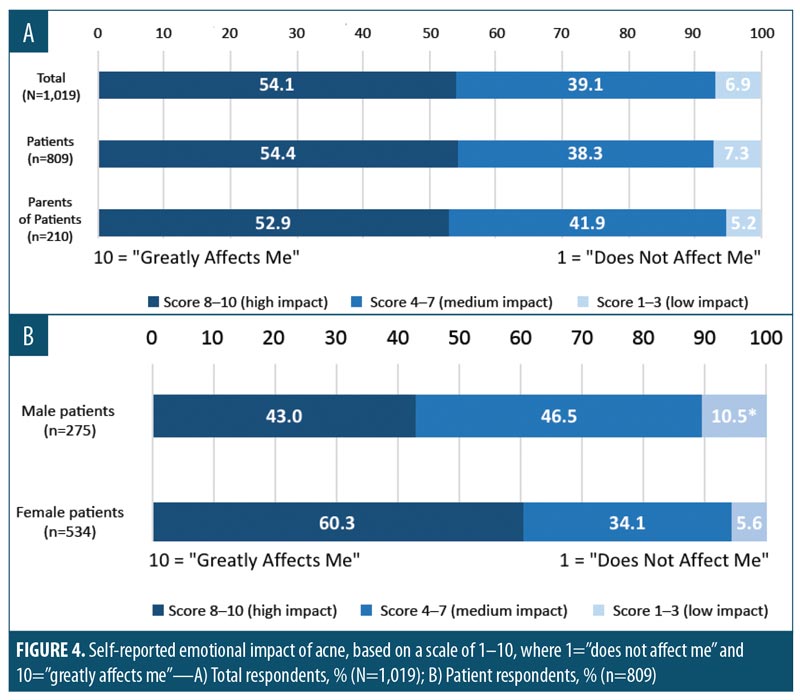
Emotional impact of acne. The majority of respondents reported that acne had an emotional impact on themselves or their children (Figure 4A), with 54.1 percent of respondents giving the impact a score of eight points or more on a 10-point scale, where 10=“affects me greatly” and 1=“does not affect me.” A greater proportion of women reported that AV had a major emotional impact in that 60.3 percent of women versus 43.0 percent of men ranked emotional impact with a score of 8 or higher (Figure 4B). Overall, 43.5 percent of those surveyed agreed with the statement, “I care more about the emotional impact of my acne today than about the potential long-term impact of antibiotic resistance.” When compared to parents, a greater percentage of patients with AV (46.7% vs. 31.0%) somewhat or completely agreed with this statement (significant at 95% CI). The difference between the two groups was driven by a low proportion of female parents displaying agreement (25.0%). A significantly greater proportion of patients, both male and female, as well as male parents agreed that the emotional impact of AV outweighs the risk of antibiotic resistance (significant at 95% CI). Of all parents surveyed, 65.7 percent reported that they had suffered from AV as a child or young adult. Of these, 47.8 percent ranked the emotional impact of AV as 8 or higher on a 10-point scale.
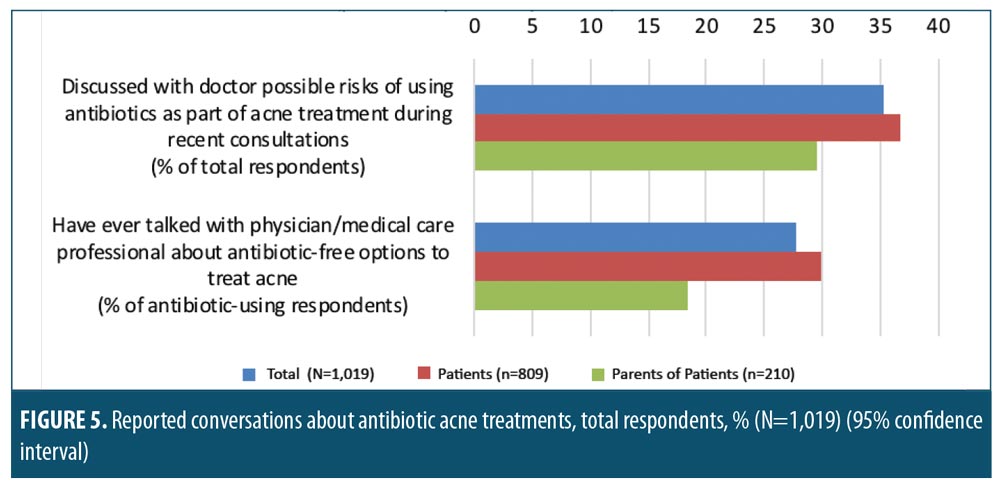
Conversations with dermatologists about antibiotic resistance related to acne. Of all respondents surveyed, only 35.3 percent had discussed the possible risks of antibiotic use as part of their AV treatment during recent conversations with their healthcare provider. These discussions were most often initiated by the doctor (65.6% of all reported discussions). Patients were more likely to report having discussed antibiotic risks with their doctor than parents were (Figure 5), and male patients were significantly more likely to report these discussions than female patients (46.9% vs. 31.6%, respectively, significant at 95% CI). Few antibiotic users (including those unsure whether they had been prescribed an antibiotic) reported having discussed antibiotic-free AV treatment options with their prescriber (27.7%). Discussions concerning antibiotic-free treatment options were significantly more likely to occur among patients than parents (Figure 5; significant at 95% CI) and significantly more likely to occur among male patients than female patients (42.6% vs. 23.8%, respectively, significant at 95% CI).
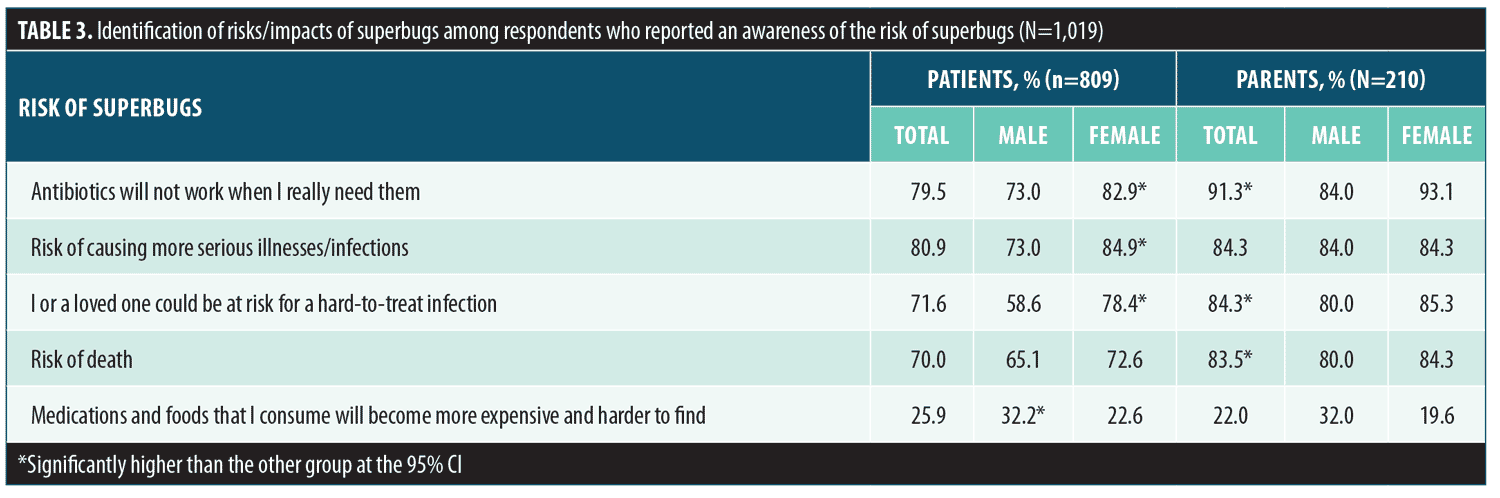
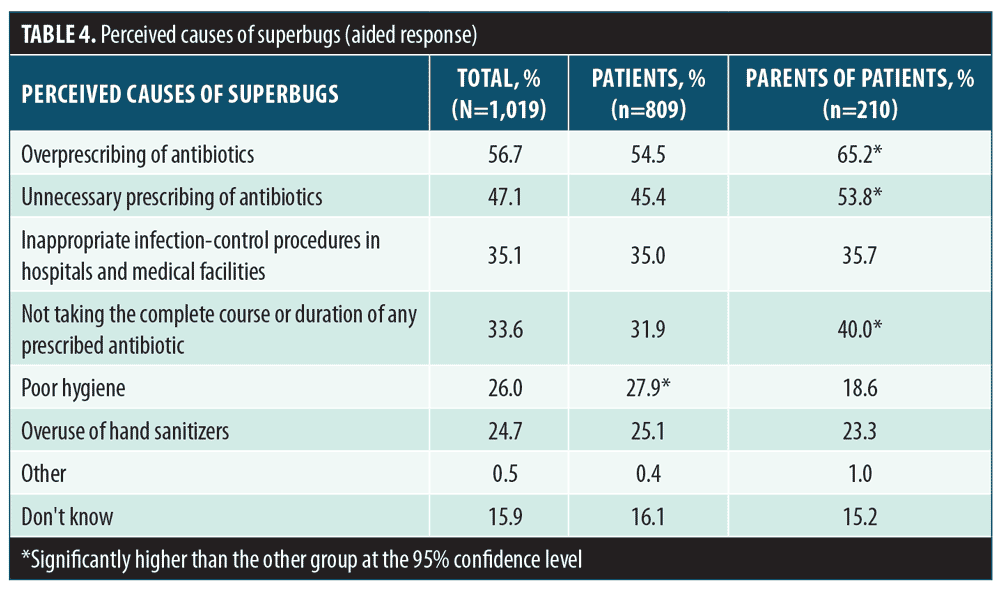
Risks and actions related to antibiotic resistance. More than half of respondents (61.5%) reported that they had heard the term superbug, with 56 percent of all respondents reporting that they were aware of the risk and impact of superbugs. Parents were significantly more likely to report an awareness of superbugs compared to patients (72.9% vs. 58.6%, respectively, significant at 95% CI). A slightly higher percentage of female patients reported an awareness of superbugs compared to male patients (60.7% vs. 54.5%, respectively). The percentages of respondents who stated they were aware of specific health risks and costs (aided response question) associated with superbugs are presented in Table 3. Significant differences between the proportion of male and female patients and between patients and parents who correctly identified superbug-related risks were observed (significant at 95% CI). The respondents perceived a myriad of contributing factors to the propagation of superbugs, with overprescribing or unnecessary prescribing of antibiotics among the most commonly selected reasons for superbugs. In general, parents were more aware of causal factors than were current patients with AV (Table 4). A significantly greater percentage of female respondents were aware of causal factors related to antibiotic resistance compared to male respondents, including overprescribing of antibiotics, unnecessary prescribing of antibiotics, and not taking the complete course or duration of any prescribed antibiotic (significant at 95% CI). A significantly greater percentage of male respondents identified poor hygiene as a contributing factor leading to the propagation of superbugs (significant at 95% CI), compared to female respondents.
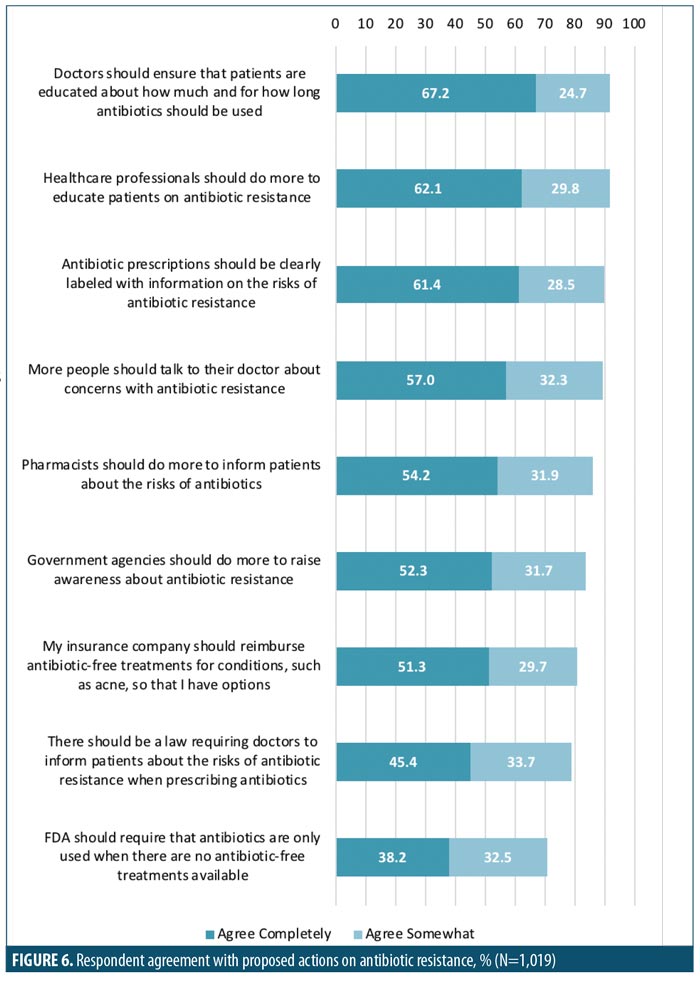
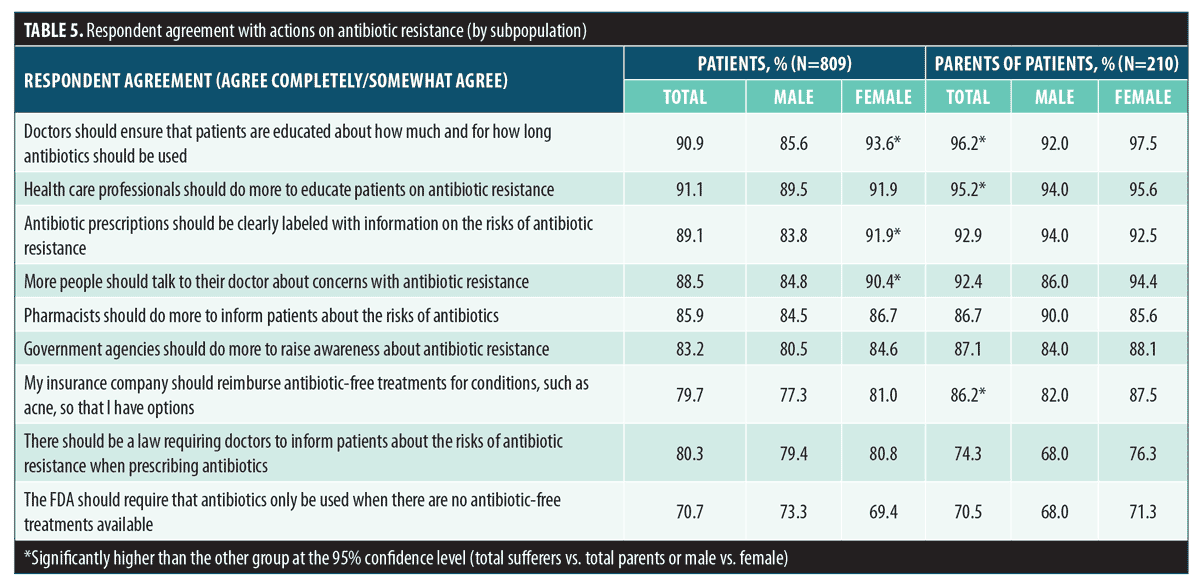
Subject agreement with a number of potential actions targeting antibiotic resistance was collected. The greatest proportion (>90%) of subjects agreed that doctors and other healthcare professionals should educate patients on antibiotic use and resistance (Figure 6). Parents were more likely than patients to agree with the proposed actions around antibiotic use, and female patients were more likely than male patients to agree with the proposed actions (significant at 95% CI; Table 5).
Discussion
This study reports the results of a survey of adults with AV and the parents of adolescents with AV. The survey assessed attitudes toward and awareness of antibiotic resistance and antibiotic use in AV. According to the results of this survey, adult patients with AV and parents of children/adolescents with AV have a basic grasp of antibiotic resistance, yet they underestimate many of the potential associated risks. Increased awareness was associated with increasing age within the surveyed population. Therefore, it might be important to focus on increasing awareness among younger patients with AV and to spend more time discussing the potential resistance-related risks of antibiotics with this population. Regarding the relative risk of antibiotic resistance, survey respondents perceived the use of oral antibiotics as a greater risk than the use of topical agents. Only a minority of respondents were aware of healthcare cost-related risks associated with superbugs, so education around the potential economic burden of superbugs (Table 3) is an area of opportunity.
The high emotional impact of AV increases the importance of efficacy in treatment decisions; however, consumers in this survey were clearly found to be open to or even prefer antibiotic-free options if the antibiotic-free treatment is effective. The results of this study demonstrate that patients with AV and parents of younger patients with AV feel that more should be done to combat antibiotic resistance, with most respondents placing the onus on healthcare professionals to educate patients on the topic. Antibiotics are a very important part of the AV therapeutic armamentarium. However, it is important that clinicians educate patients on antibiotic use and risks and incorporate exit plans when treating disorders such as AV when antibiotic therapy is prescribed.
Education surrounding antibiotic use: what more needs to be done? Dermatological disorders, including AV and rosacea, frequently have a powerful psychosocial impact. Psychosocial consequences and negative changes in emotional and psychological health and well-being have been widely reported in the literature.39,50–54 Furthermore, patients with AV can also develop scars, post-inflammatory hyperpigmentation, and erythema. These sequelae can persist for months or years after the active AV lesion that triggered them has resolved. In the present study, more than 70 percent of respondents reported that AV had a high emotional impact. A greater proportion of women than men reported that the disease had a high emotional impact. However, this patient population was also more likely to agree with statements of action regarding antibiotic resistance. Therefore, despite the need and desire for efficacious treatments for AV, survey respondents indicated that they would like more information about antibiotic-free treatment options and agreed that more should be done to raise awareness of issues related to antibiotic resistance.
Nearly 90 percent of those surveyed agreed that antibiotic prescriptions should be clearly labeled with information on the risks of antibiotic resistance. Additionally, more than 80 percent of subjects agreed that government agencies should do more to raise awareness about antibiotic resistance. The CDC has taken up this call through their “Get Smart about Antibiotics Week” initiative. This annual event aims to increase awareness and education concerning antibiotic use and antibiotic resistance. Similar programs are in place throughout the industrialized world.
Nearly all respondents surveyed agreed that healthcare professionals should be involved in efforts to educate patients about antibiotic resistance, and respondents ranked actions by healthcare professionals as being the most effective. This indicates that there is an opportunity to improve the existing dialogue between clinicians, their staff, patients with AV, and parents of patients that are minors. Notably, the results of the survey indicated a need for improved patient knowledge regarding the specific risks of antibiotic resistance, particularly healthcare cost-associated risks, and knowledge of antibiotic-free treatment options. Because the duration of antibiotic exposure is a critical factor in the propagation of antibiotic resistance, clinicians should be particularly mindful of educating patients with disorders that often require long-term treatment, such as AV. The World Health Organization has recommended that clinicians only prescribe antibiotics when they are truly needed, utilizing an appropriate agent in the correct dose and for the correct duration of therapy.14 The latter is not always clearly defined for adequate treatment of AV; however, attempts to limit prolonged oral antibiotic use are recommended.10,14 This warrants an exit plan that incorporates topical therapy for long-term control of AV or transition to oral isotretinoin therapy when indicated. An open dialogue between clinicians and/or their staff and patients about the necessity for and alternatives to antibiotic therapies is consistent with the recommendation to limit oral antibiotic prescribing.
Antibiotic-free treatment options Approximately 95 percent of patients and parents surveyed in this study did not think that AV could only be treated with antibiotics, and the majority responded that they would prefer to use an effective, antibiotic-free prescription treatment. Despite this, the awareness of antibiotic-free prescription AV treatments among antibiotic users was quite low. There are a number of effective, safe, antibiotic-free AV treatment options available to patients, and patient education on antibiotic-free treatment options is one avenue for expanding public knowledge and preventing the threat of increasing antibiotic resistance. Antibiotic-free AV treatment options are presented in Table 6.
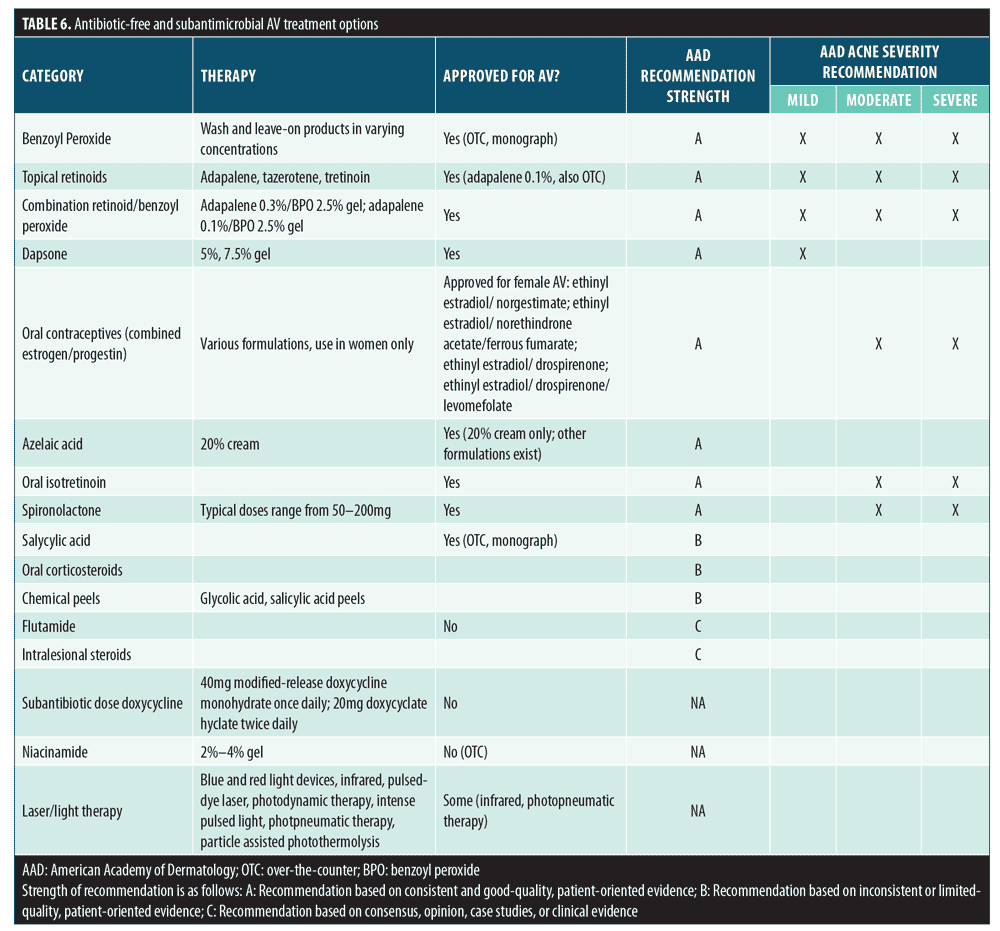
Benzoyl peroxide. In terms of bactericidal action, benzoyl peroxide (BP) provides potent antimicrobial action with no known potential for inducing bacterial resistance. BP is effective at relatively low concentrations, even in the presence of sebum.9,55–58 BP also has keratolytic properties, has been shown to reduce comedones in patients with AV , and might contribute indirectly to anti-inflammatory activity, which can also be beneficial in AV treatment.57,59,60 Due to strong bactericidal action and a lack of resistance induction, some authors have recommended that BP be added to any antibiotic treatment for AV.9,10,32 However, topical BP will not prevent alterations in systemic flora, including the development of antibiotic-resistant bacteria and changes in the GI tract microbiome, resulting from the use of oral antibiotics. Fixed-dose combination products containing BP and either clindamycin or erythromycin have been approved for the treatment of AV.61–64 It should be noted that C. acnes resistance to erythromycin is widespread, resistance to clindamycin has been observed, and cross-resistance among C. acnes strains to both antibiotics is common.65–68 The incorporation of BP in combination with a topical antibiotic (used as a fixed combination product), such as erythromycin, has been shown to prevent the emergence and proliferation of antibiotic-resistant C. acnes strains, at least at sites where the BP is applied.9,69,70
Retinoids. Retinoids are an effective alternative to antibiotic treatment for AV and are recommended for both initial and maintenance of AV by the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne.9,10,32 Retinoids have keratolytic and anti-comedogenic properties, exhibit direct anti-inflammatory activity via downregulation of Toll-like receptor-2 (TLR-2, and might contribute to dermal matrix integrity and acne scar mitigation through the stimulation of collagen production.13,14,59,71,72 Fixed-dose combinations of the retinoid adapalene with BP are also available and are an effective antibiotic-free topical option for AV treatment.9
Dapsone. Topical dapsone might serve as an antibiotic-free option for AV, though whether currently marketed topical dapsone formulations exhibit antibiotic activity and/or affects the cutaneous flora remains unknown. In vitro, dapsone demonstrates antimicrobial activity against a wide range of gram-positive bacteria, with several species (including S. aureus, Staphylococcus epidermidis [S. epidermidis], Streptococcus pyogenes [S. pyogenes], and Streptococcus agalactiae [S. agalactiae]) exhibiting a minimum inhibitory concentration (MIC)50 of 128 micrograms/mL or less.73 A topical dapsone 2% nanoemulsion has shown very high (11,963,837.34 micrograms/cm2) local skin concentrations, and therefore, might affect many commensal and pathogenic bacteria.73,74
Conclusion
Our survey results suggest that the majority of patients with AV and parents of young patients with AV are aware of and concerned about the impact of antibiotic resistance. Adult patients with AV and parents of younger patients with AV have a general understanding of the risks associated with antibiotic resistance and many of the potential causes of antibiotic resistance; however, they underestimate the role of topical treatments in the development of antibiotic resistance. Most of the respondents were not aware of antibiotic-free treatment options, but the vast majority were open to using an effective, antibiotic-free treatment for AV on themselves or their children. This study highlights an existing desire among patients with AV for more information from their clinicians about antibiotic resistance risk and alternative therapies for AV.
A full understanding of the mechanisms of action of antibiotics in AV is not known; however, the reduction of C. acnes appears to be one factor that correlates with clinical improvement, and its increasing resistance to antibiotics has been associated with reduced efficacy of antibiotic therapy for AV.75–77 Anti-inflammatory properties of some antibiotics (e.g., tetracyclines, macrolides) might also contribute to their therapeutic effects for AV.36,75,78 Moreover, while antibiotic resistance can develop quickly and is widespread, both oral and topical antibiotics are often prescribed for extended periods. This suggests that the anti-inflammatory contribution of antibiotics to AV treatment is significant.4,79–82 Subantibiotic dosing of oral antibiotics or combining topical antibiotics with BP are two potential approaches to mitigating the risk of antibiotic resistance while harnessing the anti-inflammatory effects of antibiotics.9,10,83,84 Antibiotics are widely prescribed in dermatology; therefore, judicious use of antibiotics in the treatment of noninfectious dermatological diseases (such as AV and rosacea) and increased education of dermatology patients have the potential for great impact in the stewardship of antibiotic therapies.
References
- Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187.
- Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529(7586):336–343.
- Tavernise BS, Grady D. Infection raises specter of superbugs resistant to all antibiotics. Available at: https://www.nytimes.com/2016/05/27/health/infection-raises-specter-of-superbugs-resistant-to-all-antibiotics.html. Accessed June 1, 2016.
- Del Rosso JQ, Webster GF, Rosen T. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9(4):18–24.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004. A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500.
- Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne!. Skin Therapy Lett. 2013;18(5):1–4.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatology. 2014;24(3): 330–334.
- Symphony Health PHAST Monthly Prescription.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5): 945–973.e33.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne Group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–S50.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 Pt 3):S34–S37.
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580.
- Dreno B, Gollnick HPM, Kang S, et al. Understanding innate immunity and inflammation in acne: Implications for management. J Eur Acad Dermatology Venereol. 2015;29(S4):3–11.
- Das S, Reynolds R V. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15(6):479–488.
- Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22(5):360–366.
- Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160(2):345–352.
- Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99(10):7148–7153.
- Jeremy AHT, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27.
- Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153(6):1105–1113.
- Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134(11):2747–2756.
- Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1 beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134(2):381–388.
- Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27–35.
- Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28(3):271–278.
- Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):2–7.
- Chronnell CMT, Ghali LR, Ali RS, et al. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117(5): 1120–1125.
- Nakatsuji T, Kao MC, Zhang L, et al. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010;130(4): 985–994.
- Lee DY, Yamasaki K, Rudsil J, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill Propionibacterium acnes. J Invest Dermatol. 2008;128(7):1863–1866.
- Jappe U. Pathological mechanisms of acne with special emphasis on Propionibacterium acnes and related therapy. Acta Derm Venereol. 2003;83(4):241–248.
- McDowell A, Barnard E, Nagy I, et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of “pathogenic”, “commensal” and antibiotic resistant strains. PLoS One. 2012;7(7):e41480.
- McDowell A, Nagy I, Magyari M, et al. The Opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One. 2013;8(9):e70897.
- Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013;27(1):1–10.
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663.
- Lucky AW, Cullen SI, Funicella T, et al. Double-blind, vehicle-controlled, multicenter comparison of two 0.025% tretinoin creams in patients with acne vulgaris. J Am Acad Dermatol. 2016;38(4):S24–S30.
- Richter JR, Bousema MT, Boulle K De, et al. Efficacy of a fixed clindamycin phosphate 1.2%, tretinoin 0.025% gel formulation (Velac) in the topical control of facial acne lesions. J Dermatolog Treat. 1998;9(2):81–90.
- Gollnick HPM, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161(5):1180–1189.
- Stein-Gold LF, Cruz A, Eichenfield L, et al. Effective and safe combination therapy for severe acne vulgaris: a randomized, vehicle-controlled, double-blind study of adapalene 0.1%-benzoyl peroxide 2.5% fixed-dose combination gel with doxycycline hyclate 100mg. Cutis. 2010;85(2):94–104.
- Thiboutot DM, Weiss J, Bucko A, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57(5):791–799.
- Stein-Gold L, Weiss J, Rueda MJ, et al. Moderate and severe inflammatory acne vulgaris effectively treated with single-agent therapy by a new fixed-dose combination adapalene 0.3 %/benzoyl peroxide 2.5 % gel: a randomized, double-blind, parallel-group, controlled study. Am J Clin Dermatol. 2016; 17(3):293–303.
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7(5):281–290.
- Leccia MT, Auffret N, Poli F, et al. Topical acne treatments in Europe and the issue of antimicrobial resistance. J Eur Acad Dermatol Venereol. 2015;29(8):1485–1492.
- Del Rosso JQ, Gallo RL, Thiboutot D, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 2: perspectives on antibiotic use and the microbiome and review of microbiologic effects of selected specific therapeutic agents commonly Used by dermatologists. J Clin Aesthet Dermatol. 2016;9(5):11–7.
- Del Rosso JQ, Rosen T, Thiboutot D, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 3: current perspectives on skin and soft tissue infections with emphasis on methicillin-resistant Staphylococcus aureus, commonly encountered scenarios when antibiotic use may not be needed, and concluding remarks on rational use of antibiotics in dermatology. J Clin Aesthet Dermatol. 2016;9(6):17–24.
- Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–167.
- Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169.
- Blaser M. Stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–394.
- Vowels BR, Feingold DS, Sloughfy C, et al. Effects of topical erythromycin on ecology of aerobic cutaneous bacterial flora. Antimicrob Agents Chemother. 1996;40(11):2598–2604.
- Mills O, Thornsberry C, Cardin CW, et al. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002;82(4):260–265.
- United States Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed June 1, 2016.
- World Health Organization. Global report on surveillance: antimicrobial resistance 2014. Available at: https://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed June 1, 2016.
- Gupta MA, Johnson AM, Gupta AK. The development of an acne quality of life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78(6):451–456.
- Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol. 1998;139(5):846–850.
- Rubinow DR, Peck GL, Squillace KM, Gantt GG. Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J Am Acad Dermatol. 1987;17(1): 25–32.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
- Koo J. The psychosocial impact of acne: patients’ perceptions. J Am Acad Dermatol. 1995;32(5 Pt 3):S26–S30.
- Bojar RA, Cunliffe WJ, Holland KT. The short-term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora. Br J Dermatol. 1995;132(2):204–208.
- Pannu J, McCarthy A, Martin A, Hamouda T, et al. In vitro antibacterial activity of NB-003 against Propionibacterium acnes. Antimicrob Agents Chemother. 2011;55(9):4211–4217.
- Hegemann L, Toso SM, Kitay K, Webster GF. Anti-inflammatory actions of benzoyl peroxide: Effects on the generation of reactive oxygen species by leucocytes and the activity of protein kinase C and calmodulin. Br J Dermatol. 1994;130(5):569–575.
- Mills OH, Kligman AM, Pochi P, Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. Int J Dermatol. 1986;25(10):664–667.
- Zuliani T, Khammari A, Chaussy H, Knol AC, et al. Ex vivo demonstration of a synergistic effect of Adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20(10):850–853.
- Waller JM, Dreher F, Behnam S, et al. “Keratolytic” properties of benzoyl peroxide and retinoic acid resemble salicylic acid in man. Skin Pharmacol Physiol. 2006;19(5):283–289.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37(4):590–595.
- Leyden JJ, Hickman JG, Jarratt MT, et al. The efficacy and safety of a combination benzoyl peroxide/clindamycin topical gel compared with benzoyl peroxide alone and a benzoyl peroxide/erythromycin combination product. J Cutan Med Surg. 2001;5(1):37–42.
- Pariser DM, Rich P, Cook-Bolden FE, Korotzer A. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate-to-severe acne vulgaris. J Drugs Dermatol. 2014;13(9): 1083–1089.
- Tschen EH, Katz HI, Jones TM, et al. A combination benzoyl peroxide and clindamycin topical gel compared with benzoyl peroxide, clindamycin phosphate, and vehicle in the treatment of acne vulgaris. Cutis. 2001;67(2):165–169.
- Dumont-Wallon G, Moyse D, Blouin E, Dréno B. Bacterial resistance in French acne patients. Int J Dermatol. 2010;49(3):283–288.
- Schafer F, Fich F, Lam M, et al. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52(4):418–425.
- Mendoza N, Hernandez PO, Tyring SK, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52(6):688–692.
- Crawford WW, Crawford IP, Stoughton RB, Cornell RC. Laboratory induction and clinical occurrence of combined clindamycin and erythromycin resistance in Corynebacterium acnes. J Invest Dermatol. 1979;72(4):187–190.
- Eady E a, Bojar R a, Jones CE, et al. The effects of acne treatment with a combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin-resistant propionibacteria. Br J Dermatol. 1996;134(1):107–113.
- Eady EA, Farmery MR, Ross JI, et al. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic-sensitive and -resistant skin bacteria from acne patients. Br J Dermatol. 1994;131(3):331–336.
- Jalian HR, Liu PT, Kanchanapoomi M, Phan JN, et al. All-trans retinoic acid shifts Propionibacterium acnes–induced matrix degradation expression profile toward matrix preservation in human monocytes. J Invest Dermatol. 2008;128(12):2777–2782.
- Czernielewski J, Michel S, Bouclier M, Baker M. Adapalene biochemistry and the evolution of a new topical retinoid for treatment of acne. J Eur Acad Dermatol Venereol. 2001;15 Suppl 3:5–12.
- Zhanel GG, Del Rosso JQ. Activity of dapsone versus community and hospital pathogens from the CANWARD study. J Clin Aesthet Dermatol. 2016;9(3):42–47.
- Borges VR, Simon A, Sena ARC, et al. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int J Nanomedicine. 2013;8:535–544.
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467–478.
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121(1):51–57.
- Ozolins M, Eady EA, Avery AJ, et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet. 1997;364(9452):2188–2195.
- Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24(7):1117–1133.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153(2):395–403.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11(12):1434–1438.
- Walker C, Bradshaw M, editors. The effect of oral doxycycline 100 mg once-daily for 14 days on the nasopharyngeal flora of healthy volunteers: a preliminary analysis. Poster presented at the 26th Anniversary Fall Clinical Dermatology Conference; October 18–27, 2007; Las Vegas, NV.
- Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54(2):258–265.
- Toossi P, Farshchian M, Malekzad F, Mohtasham N. Subantimicrobial-dose doxycycline in the treatment of moderate facial acne. J Drugs Dermatol. 2008;7(12):1149–1152.
- Moore A, Ling M, Bucko A, et al. Efficacy and safety of subantimicrobial dose, modified-release doxycycline 40 mg versus doxycycline 100 mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: a randomized, double-blinded, controlled study. J Drugs Dermatol. . 2015;14(6):581–586.

